|
What is an interaction?
Most engineers and physical scientists working today
were educated in the school of experimentation which dictates that we vary one
factor at a time. In this way, so
the theory goes, we can isolate the effect of each factor without contaminating
our results with the effects of other factors.
However, a process involving many factors will usually be more complex
than this. For example, if we
determine the optimum point for one factor, how can we be sure that it will
remain the optimum point at all levels of the other factors? This last point is one of the most powerful features of
Design of Experiments (DoE).
If the optimum setting of a factor is dependent on the setting of
another factor then we say that there is an interaction between the two
factors.
The
one-factor-at-a-time approach cannot give you this
crucial information on interactions.
Consider a simple example where we have a chemical
process in which the yield is dependent on the temperature and the
concentration of catalyst. If we
ran an experiment using the one-factor-at-a-time approach then measurements
would first be taken with, say, the concentration held constant and the
temperature varied in appropriate steps. This
procedure would then be repeated with the temperature constant and the
concentration varying. We would
then be able to construct two plots, one showing the variation of yield versus
temperature and the other showing the variation with catalyst concentration.
In considering the yield versus temperature we have
to ask the important question: Will
the plot of yield versus temperature always be the same, irrespective of which
concentration is used? If this is
not the case, i.e. the relationship between the yield and temperature changes,
then we say that we have an interaction between the two factors, temperature
and concentration.
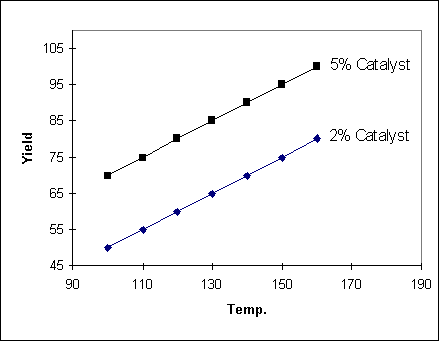
Example 1
In the following example, the
change in yield with temperature does not depend on the catalyst setting
(and so the two lines are parallel). Another way of saying this is that there
is no interaction between Temperature and Catalyst. In such a case the yield
can be described by the equation :
Yield = aT + bC where a and b
are constants and T and C are the temperature and catalyst settings
respectively.
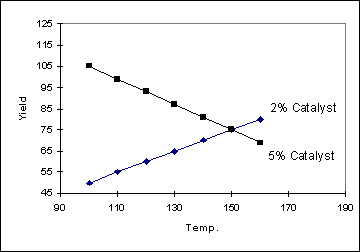
Example 2
In the following example, the
change in yield with temperature does depend on the catalyst setting
(and so the two lines are not parallel). Another way of saying this is that
there is an interaction between Temperature and Catalyst. The more non-parallel
the lines are, the stronger the interaction is. When an interaction is present
between the factors, the yield can be described by the equation :
Yield = aT + bC + cTC where a,
b and c are constants and T and C are the temperature and catalyst settings
respectively.
The interaction constant, c,
can be positive or negative.
The potential problems
associated with the presence of interactions can be very clearly seen when we
view the resulting response surface in three dimensions.
|